Background
Chimeric antigen receptor (CAR) T-celltherapy has become the standard of care for many relapsed/refractory hematological malignancies. There are significant associated side effects, most importantly cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) that can limit its use in older and vulnerable populations. The rate of ICANS with axicabtagene ciloleucel (axi-cel) in the pivotal studies for relapsed/refractory diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) was approximately 60% with grade 3 or higher (≥ G3) in the range of 19-28%. Similarly, with brexucabtagene autoleucel (brexu-cel), ICANS also occurred in approximately 60% with ≥ G3 occurring in 26-31% of patients. The mechanism underlying ICANS remains unclear. The current hypothesis is that endothelial injury caused by systemic immune activation leads to the breakdown of the blood-brain barrier with the influx of pro-inflammatory cytokines into the CSF and subsequent activation of resident immune cells and neuroglial cells leading to local production of cytokines and the clinical syndrome observed. ICANS is treated with systemic steroids that have good central nervous system (CNS) penetration. Statins have been shown to stabilize the endothelium and have anti-inflammatory effects. We are currently evaluating the safety and feasibility of administering simvastatin in addition to intrathecal dexamethasone to decrease the incidence and/or severity of neurotoxicity in CAR-T recipients and we are herein reporting preliminary results.
Methods
This is a feasibility study (NCT04514029) combining simvastatin 40 mg daily, starting 5 days prior to apheresis and continuing through day +30 with dexamethasone (8 mg) delivered intrathecally (IT) on days -1 and +6 in relation to CAR T-cell infusion. Adult patients (age ≥18 years) receiving axi-cel or brexu-cel for FDA-approved indications are included. The primary endpoint is the percentage of patients who complete 80% of this therapy (pills and intrathecal). Secondary endpoints include the incidence of CRS and ICANS as well as overall response rates (ORR).
Results
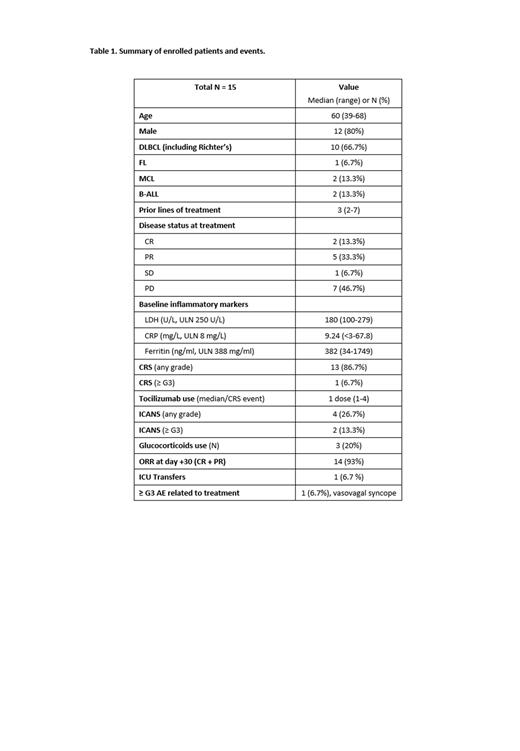
At the time of data cutoff, a total of 15 patients who received treatment with axi-cel or brexu-cel and completed follow-up were enrolled. Baseline demographics and clinical characteristics are summarized in Table 1. Among patients who received axi-cel, 10 patients had DLBCL and 1 patient had FL. For brexu-cel, 2 patients had mantle cell lymphoma (MCL) and 2 had B-acute lymphoblastic leukemia (B-ALL). Median prior lines of treatment was 3 (range 2 to 7) and 11 patients (46.7%) had progressive disease at the time of the treatment. Analysis of baseline inflammatory markers prior to the cell infusion showed that 2 patients (13.3%) had elevated lactate dehydrogenase (LDH; upper limit normal 250 U/L; median 180, range 100 to 279). Eight patients (53.3%) had elevated C-reactive protein (CRP; median 9.24 mg/L, range <3 to 67.8). Ferritin was elevated in 6 patients (40%) (median 382, range 34 to 1749). All patients received > 80% of the total assigned doses. One patient was unable to receive intrathecal therapy due to thrombocytopenia with platelet transfusion refractoriness. All evaluable patients completed oral simvastatin with no patients requiring dose reductions. CRS occurred in 13 patients (86.7%), with ≥ G3 occurring in 1 patient (6.7%). Tocilizumab (8 mg/kg) was administered to 11 patients (73.3%) with the median number of doses being 1 (range 1 to 4). ICANS occurred in 4 patients (26.7%), with ≥ G3 occurring in 2 patients (13.3%) - one G3 and one G4 in the patient who was unable to receive IT therapy. Glucocorticoids were used in 3 patients (20%) for the management of ICANS. There were no grade 4 CRS events and no deaths related to CRS or ICANS. One patient had a vasovagal syncopal episode prior to the beginning of a lumbar puncture. There were no other ≥ G3 AEs associated with the administration of simvastatin or IT dexamethasone. One patient with Richter's had progressive disease at day 30, 4 patients had partial remission, and 10 patients had a complete remission for an ORR of 93%.
Conclusions
Preliminary results show that simvastatin and intrathecal dexamethasone appear to be feasible, safe, and effective in reducing the incidence and severity of ICANS in patients receiving axi-cel and brexu-cel.
OffLabel Disclosure:
Hu:AbbVie: Membership on an entity's Board of Directors or advisory committees. Holtan:Vitrac: Research Funding; Incyte: Research Funding; Ossium: Consultancy; Sanofi: Research Funding; CSL Behring: Other: Endpoint Adjudication Committee. Betts:CRISPR: Patents & Royalties; Incyte: Membership on an entity's Board of Directors or advisory committees; CTI Biopharma: Research Funding; Vitrac Therapeutics: Research Funding. Bachanova:BMS: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Miltenyi: Other: DSMB; Incyte: Research Funding; Citius: Research Funding; Gamida Cell: Research Funding; Allogene: Membership on an entity's Board of Directors or advisory committees; ADC: Membership on an entity's Board of Directors or advisory committees. Miller:GT BioPharma: Consultancy, Current holder of stock options in a privately-held company, Patents & Royalties, Research Funding; Vycellix: Consultancy, Current holder of stock options in a privately-held company; Sanofi: Membership on an entity's Board of Directors or advisory committees; Fate Therapeutics: Consultancy, Current holder of stock options in a privately-held company, Patents & Royalties, Research Funding. Maakaron:Precision Biosciences: Research Funding; Gilead: Research Funding; Atara: Research Funding; CRISPR: Research Funding; FATE Therapeutics: Research Funding; CLBR: Research Funding.
- Simvastatin - Anti-inflammatory. Prevention of endothelial injury leading to CAR T-cell related neurotoxicity. - Intrathecal dexamethasone - Anti-inflammatory. Prevention of CAR T-cell related neurotoxicity.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal